HSP70/HSPA1 Mouse mAb [76VH]Cat NO.: A75114
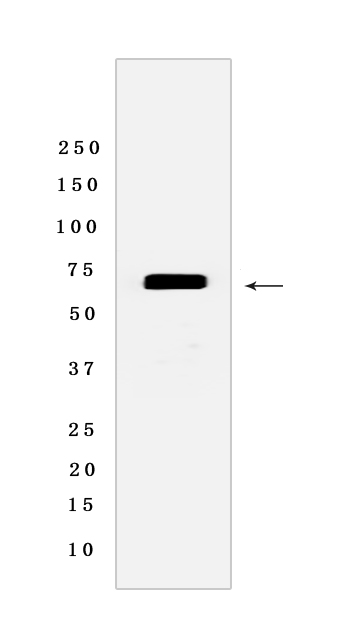
Western blot(SDS PAGE) analysis of extracts from HepG2 cells.Using HSP70/HSPA1 Mouse mAb [76VH] at dilution of 1:1000 incubated at 4℃ over night.
Product information
Protein names :HSPA1A,HSP72,HSPA1,HSX70,HS71A_HUMAN,Heat shock 70 kDa protein 1A
UniProtID :P0DMV8
MASS(da) :70,052
MW(kDa) : 70kDa
Form :Liquid
Purification :Protein A purification
Host :Mouse monoclonal IgG
Isotype :IgG
sensitivity :Endogenous
Reactivity :Human,Mouse
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with a synthetic peptide of human HSP70/HSPA1.
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 50% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :HepG2 cells
Function : Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1 (PubMed:24012426, PubMed:26865365, PubMed:24318877). Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation (PubMed:27708256). Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle (PubMed:27137183). Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling (PubMed:24613385). Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation (PubMed:23973223). Negatively regulates heat shock-induced HSF1 transcriptional activity during the attenuation and recovery phase period of the heat shock response (PubMed:9499401). Involved in the clearance of misfolded PRDM1/Blimp-1 proteins. Sequesters them in the cytoplasm and promotes their association with SYNV1/HRD1, leading to proteasomal degradation (PubMed:28842558).., (Microbial infection) In case of rotavirus A infection, serves as a post-attachment receptor for the virus to facilitate entry into the cell..
Subcellular locationi :Cytoplasm. Nucleus. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome. Secreted.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.